
En la última década, hemos asistido a una revolución en el tratamiento del cáncer de próstata resistente a la castración metastásico (CPRCm), una forma avanzada y letal de cáncer de próstata. Gracias al mayor conocimiento de sus características biológicas subyacentes, se ha podido desarrollar una serie de compuestos dirigidos a la vía de señalización andrógena y el sistema inmunitario, así como taxanos y radiofármacos.
Nuestro modelo de investigación sigue un planteamiento del laboratorio a la práctica y viceversa. Hemos creado una plataforma para adquirir muestras longitudinales de pacientes con cáncer de próstata avanzado, tanto tejido tumoral como biopsias líquidas, que puedan utilizarse para investigar las características evolutivas de la enfermedad, así como para generar modelos de laboratorio de cáncer de próstata avanzado derivados de pacientes que podamos aprovechar para investigar nuevas estrategias terapéuticas en el laboratorio.
Además, la puesta en marcha de ensayos clínicos iniciados por investigadores es fundamental para nuestra estrategia de investigación como plataforma para estudios correlativos que puedan optimizar la vía de desarrollo de fármacos para pacientes con cáncer de próstata. Actualmente, somos el laboratorio central de dos ensayos clínicos multicéntricos académicos, y también actuamos como repositorio nacional del registro IRONMAN, una importante iniciativa internacional para crear un gran banco de datos clínicos y bioespecímenes de pacientes con cáncer de próstata metastásico (CPm).
Nuestro objetivo es integrar conocimientos de biología molecular, genómica, transcriptómica, ciencias computacionales y datos clínicos para desarrollar estrategias de medicina de precisión para pacientes con cáncer de próstata. Para ello, nuestro equipo está formado por científicos biomédicos expertos en biología del cáncer, genómica y transcriptómica, bioinformática y biopsia líquida, así como por oncólogos médicos y gestores de datos clínicos.
Una de nuestras principales líneas de investigación es descifrar cómo se adapta el cáncer de próstata a la exposición a tratamientos sistémicos, en particular los fármacos dirigidos a los andrógenos, con especial atención a la regulación del ciclo celular y la respuesta al daño del ADN y la aparición de fenotipos quiescentes y senescentes que pueden impulsar la resistencia a los fármacos. Mediante el estudio de modelos in vitro e in vivo, incluidos los xenoinjertos derivados de pacientes (PDX) de los pacientes que participan en nuestros estudios clínicos, pretendemos atacar los fenotipos emergentes mediante combinaciones de fármacos. En la actualidad, estos estudios están financiados con subvenciones del Ministerio de Sanidad español, la Fundación FERO, la Asociación Española Contra el Cáncer (AECC) y a través de colaboraciones con empresas biofarmacéuticas.
Nuestro grupo también persigue la caracterización molecular del cáncer de próstata avanzado, centrándose en cómo los tumores evolucionan de forma heterogénea a medida que adquieren resistencia a distintos tratamientos. Por ello, hemos creado una plataforma de genómica y transcriptómica en el laboratorio para elaborar perfiles de ADN y ARN a partir de biopsias de pacientes. Estamos desarrollando nuevos ensayos de biopsia líquida que nos permitirán estudiar la enfermedad a través de muestras longitudinales. Mediante la explotación de conjuntos de datos genómicos de acceso público de pacientes en distintos estadios de la enfermedad, nuestros científicos computacionales investigan cómo cambia el perfil genómico de la enfermedad a lo largo del tiempo. En la actualidad, estos estudios están financiados por subvenciones de los Programas de Investigación Médica Dirigidos por el Congreso (CDMRP) del Departamento de Defensa de los Estados Unidos (DoD), el Ministerio de Sanidad español, la Fundación CRIS contra el Cáncer, la AECC, la Fundación FERO y la Fundación la Caixa.
Por último, dado que los datos clínicos correlativos bien comentados son cruciales para establecer la posible relevancia de los datos moleculares, nuestro equipo mantiene bases de datos que recogen los datos de resultados de todos los pacientes que donan muestras para nuestros estudios con el fin de hacer análisis correlativos posteriores.
Para avanzar más rápidamente en este campo, creemos en la ciencia de equipo. Por ello, participamos en diversas colaboraciones que aúnan recursos y combinan conocimientos transfronterizos de varios equipos y grupos de investigación.
En resumen, nuestra investigación integra diferentes modalidades de datos generados en el laboratorio que pueden llevar al diseño de intervenciones terapéuticas destinadas a mejorar los resultados de los pacientes con cáncer de próstata.
- Investigar las correlaciones entre el perfil molecular del paciente y el resultado clínico que puedan guiar estrategias de tratamiento del cáncer de próstata más precisas.
- Estudiar cómo los cánceres de próstata se adaptan a la terapia, centrándose en las vulnerabilidades emergentes para retrasar la recurrencia de la enfermedad.
- Investigar la heterogeneidad del tumor en respuesta a la terapia en modelos de xenoinjerto (PDX) preclínicos y derivados del paciente, así como interrogando biopsias de pacientes.
- Desarrollar nuevas herramientas de biopsia líquida que puedan usarse para monitorear la evolución del tumor.
- Aplicar canalizaciones computacionales para investigar las firmas genómicas en el cáncer de próstata.
- Desarrollo de ensayos clínicos académicos para validar nuestros resultados de laboratorio en la clínica y aprovechar las biopsias de los pacientes para estudios correlativos.
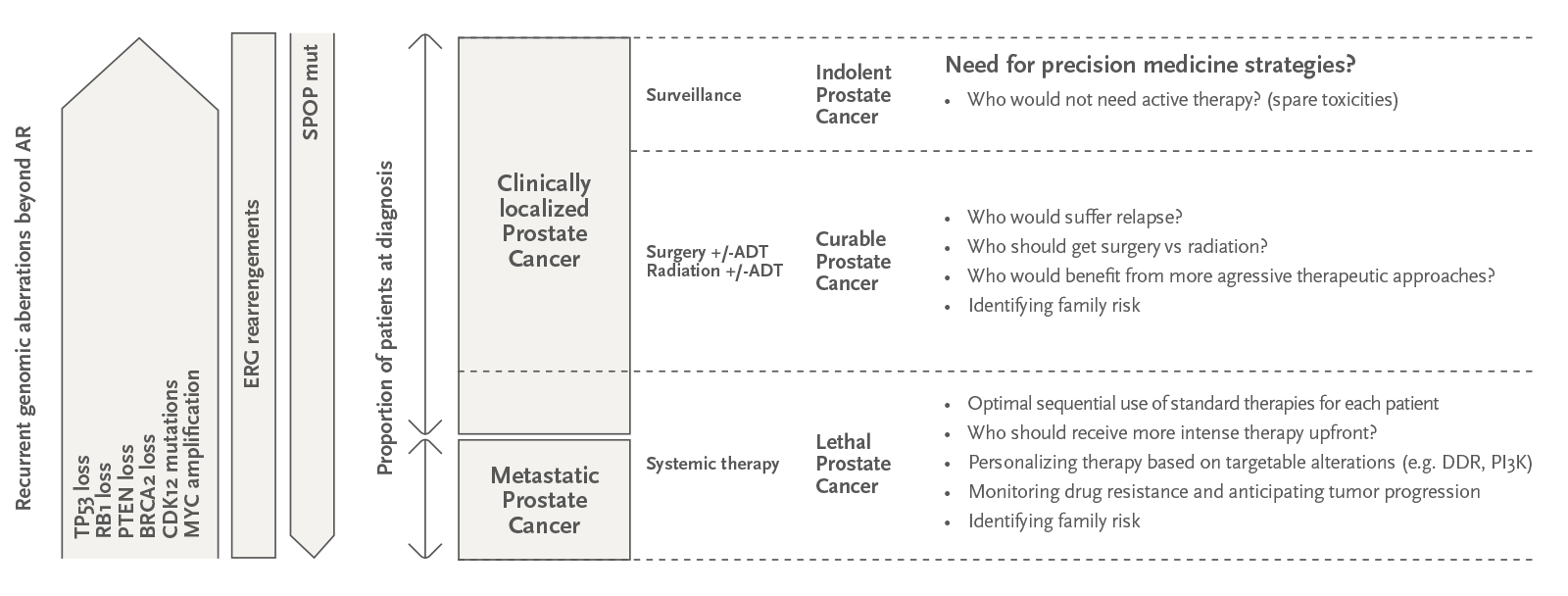
Figura: El cáncer de próstata letal tiene un paisaje molecular distinto al del cáncer de próstata localizado y curable. Un mayor conocimiento del valor pronóstico y predictivo de los distintos biomarcadores genómicos en los diferentes estados clínicos de la enfermedad puede ayudar a avanzar en muchas de las necesidades clínicas no cubiertas de los pacientes con cáncer de próstata, enumeradas en la columna derecha de la figura. Adaptado de Mateo et al., Nature Cancer, 2020.
Jefe de grupo
Joaquin Mateo
Investigador sénior
Nicolas Herranz
Becaria postdoctoral
Irene Casanova
Luisa Delgado
Becario Investigación Clínica
Pablo Cresta
Estudiantes de doctorado
Sara Arce
Julian Brandariz
Técnicas
Teresa Casals
Sarai Cordoba
Laura Agundez
Lara de Llobet
Andrei Salca
Conservadora de datos clínicos
Magdalena Guardiola
Bioinformático
Daniel Aguilar
Estudiante de máster
Arnau Solé
Publicaciones científicas más relevantes
- Zurita AJ, Graf RP, Villacampa G, Raskina K, Sokol E, Jin D, Antonarakis ES, Li G, Huang RSP, Casanova-Salas I, Vivancos A, Carles J, Ross JS, Schrock AB, Oxnard GR, Mateo J. Genomic Biomarkers and Genome-Wide Loss-of-Heterozygosity Scores in Metastatic Prostate Cancer Following Progression on Androgen-Targeting Therapies. JCO Precis Oncol. 2022 Jul;6:e2200195.
- Cresta Morgado P, Mateo J. Clinical implications of homologous recombination repair mutations in prostate cancer. Prostate. 2022 Aug;82 Suppl 1:S45-S59.
- Westphalen CB, Fine AD, André F, Ganesan S, Heinemann V, Rouleau E, Turnbull C, Garcia Palacios L, Lopez JA, Sokol ES, Mateo J. Pan-cancer Analysis of Homologous Recombination Repair-associated Gene Alterations and Genome-wide Loss-of-Heterozygosity Score. Clin Cancer Res. 2022 Apr 1;28(7):1412-1421.
- Mateo J, Steuten L, Aftimos P, André F, Davies M, Garralda E, Geissler J, Husereau D, Martinez-Lopez I, Normanno N, Reis-Filho JS, Stefani S, Thomas DM, Westphalen CB, Voest E. Delivering precision oncology to patients with cancer. Nat Med. 2022 Apr;28(4):658-665.
- Casanova-Salas I, Athie A, Boutros PC, Del Re M, Miyamoto DT, Pienta KJ, Posadas EM, Sowalsky AG, Stenzl A, Wyatt AW, Mateo J. Quantitative and Qualitative Analysis of Blood-based Liquid Biopsies to Inform Clinical Decision-making in Prostate Cancer. Eur Urol. 2021 Jun;79(6):762-771.
- Neeb A, Herranz N, Arce-Gallego S, Miranda S, Buroni L, Yuan W, Athie A, Casals T, Carmichael J, Rodrigues DN, Gurel B, Rescigno P, Rekowski J, Welti J, Riisnaes R, Gil V, Ning J, Wagner V, Casanova-Salas I, Cordoba S, Castro N, Fenor de la Maza MD, Seed G, Chandran K, Ferreira A, Figueiredo I, Bertan C, Bianchini D, Aversa C, Paschalis A, Gonzalez M, Morales- Barrera R, Suarez C, Carles J, Swain A, Sharp A, Gil J, Serra V, Lord C, Carreira S, Mateo J, de Bono JS. Advanced Prostate Cancer with ATM Loss: PARP and ATR Inhibitors. Eur Urol. 2021 Feb;79(2):200-211.
- Carreira S, Porta N, Arce-Gallego S, Seed G, Llop-Guevara A, Bianchini D, Rescigno P, Paschalis A, Bertan C, Baker C, Goodall J, Miranda S, Riisnaes R, Figueiredo I, Ferreira A, Pereira R, Crespo M, Gurel B, Nava Rodrigues D, Pettitt SJ, Yuan W, Serra V, Rekowski J, Lord CJ, Hall E, Mateo J, de Bono JS. Biomarkers Associating with PARP Inhibitor Benefit in Prostate Cancer in the TOPARP-B Trial. Cancer Discov. 2021 Nov;11(11):2812-2827.
- Westphalen B, Fine AD, Andre F, Ganesan S, Heinemann V, Rouleau E, Turnbull C, Garcia Palacios L, Lopez JA, Sokol ES, Mateo J. Pan-cancer Analysis of Homologous Recombination Repair-associated Gene Alterations and Genome-wide Loss of Heterozygosity Score. Clin Cancer Res. 2021 Nov 5:clincanres.2096.2021. Epub ahead of print.
- Casanova-Salas I, Athie A, Boutros PC, Del Re M, Miyamoto DT, Pienta KJ, Posadas EM, Sowalsky AG, Stenzl A, Wyatt AW, Mateo J. Quantitative and Qualitative Analysis of Blood-based Liquid Biopsies to Inform Clinical Decision-making in Prostate Cancer. Eur Urol. 2021 Jun;79(6):762-771. doi: 10.1016/j.eururo.2020.12.037. Epub 2021 Jan 7
- Rescigno P, Gurel B, Pereira R, Crespo M, Rekowski J, Rediti M, Barrero M, Mateo J, Bianchini D, Messina C, Fenor de la Maza MD, Chandran K, Carmichael J, Guo C, Paschalis A, Sharp A, Seed G, Figueiredo I, Lambros M, Miranda S, Ferreira A, Bertan C, Riisnaes R, Porta N, Yuan W, Carreira S, de Bono JS. Characterizing CDK12-Mutated Prostate Cancers. Clin Cancer Res. 2021 Jan 15;27(2):566-574. doi: 10.1158/1078-0432.CCR-20-2371. Epub 2020 Sep 28
- Hussain M, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, Thiery-Vuillemin A, Twardowski P, Roubaud G, Özgüroğlu M, Kang J, Burgents J, Gresty C, Corcoran C, Adelman CA, de Bono J; PROfound Trial Investigators. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020 Dec 10;383(24):2345-2357. doi: 10.1056/NEJMoa2022485. Epub 2020 Sep 20
- de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, Thiery-Vuillemin A, Twardowski P, Mehra N, Goessl C, Kang J, Burgents J, Wu W, Kohlmann A, Adelman CA, Hussain M. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020 May 28;382(22):2091-2102. doi: 10.1056/NEJMoa1911440. Epub 2020 Apr 28.PMID: 32343890
- Neeb A, Herranz N, Arce-Gallego S, Miranda S, Buroni L, Yuan W, Athie A, Casals T, Carmichael J, Rodrigues DN, Gurel B, Rescigno P, Rekowski J, Welti J, Riisnaes R, Gil V, Ning J, Wagner V, Casanova-Salas I, Cordoba S, Castro N, Fenor de la Maza MD, Seed G, Chandran K, Ferreira A, Figueiredo I, Bertan C, Bianchini D, Aversa C, Paschalis A, Gonzalez M, Morales- Barrera R, Suarez C, Carles J, Swain A, Sharp A, Gil J, Serra V, Lord C, Carreira S, Mateo J, de Bono JS. Advanced Prostate Cancer with ATM Loss: PARP and ATR Inhibitors. Eur Urol. 2021 Feb;79(2):200-211.
- Mateo J, Porta N, Bianchini D, McGovern U, Elliott T, Jones R, Syndikus I, Ralph C, Jain S, Varughese M, Parikh O, Crabb S, Robinson A, McLaren D, Birtle A, Tanguay J, Miranda S, Figueiredo I, Seed G, Bertan C, Flohr P, Ebbs B, Rescigno P, Fowler G, Ferreira A, Riisnaes R, Pereira R, Curcean A, Chandler R, Clarke M, Gurel B, Crespo M, Nava Rodrigues D, Sandhu S, Espinasse A, Chatfield P, Tunariu N, Yuan W, Hall E, Carreira S, de Bono JS. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020 Jan;21(1):162-174.
- Mateo J, Cheng HH, Beltran H, DollingD, XuW, Pritchard CC, MossopH, RescignoP, Perez-LopezR, SailerV, Kolinsky M, BalasopoulouA, BertanC, NanusDM, TagawaST, ThorneH, MontgomeryB, CarreiraS, Shahneen Sandhu S, RubinMA, Nelson PS, de BonoClinical outcome of prostate cancer patients with germline DNA repair mutations: follow-up from an international study. Eur Orol. 2018 doi:10.1016/j.euro.2018.01.010
- Mateo J, Chakravarty D, Dienstmann R, Jezdic S, Gonzalez-Perez A, Lopez-Bigas N, Ng CKY, Bedard PL, Tortora G, Douillard JY, Van Allen EM, Schultz N, Swanton C, André F, Pusztai L. A framework to rank genomic alterations as targets for cancer precision medicine: The ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol.2018 Sep 1;29(9):1895-1902.
- Rodrigues DN, Rescigno P, Liu D, Yuan W, Carreira S, Lambros MB, Seed G, Mateo J, Riisnaes R, Mullane S, Margolis C, Miao D, Miranda S, Dolling D, Clarke M, Bertan C, Crespo M, Boysen G, Ferreira A, Sharp A, Figueiredo I, Keliher D, Aldubayan S, Burke KP, Sumanasuriya S, Fontes MS, Bianchini D, Zafeiriou Z, Mendes LST, Mouw K, Schweizer MT, Pritchard CC, Salipante S, Taplin ME, Beltran H, Rubin MA, Cieslik M, Robinson D, Heath E, Schultz N, Armenia J, Abida W, Scher H, Lord C, D’Andrea A, Sawyers CL, Chinnaiyan AM, Alimonti A, Nelson PS, Drake CG, Van Allen EM, de Bono JS. Immunogenomic analyses associate immunological alterations with mismatch repair defects in prostate cancer. J Clin Invest. 2018 Nov 1;128(11):5185.
- Mateo J, Carles J. Towards a New Classification for Metastatic Prostate Cancer. Eur Urol. 2019 Mar;75(3):383-384. Epub 2018 Nov 19.
Joaquin Mateo, Gopinath Ganji , Charlotte Lemech , Howard A. Burris , Sae-Won Han , Karen Swales et al. A first-time-in-human study of GSK2636771, a phosphoinositide 3 kinase beta-selective inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2017 Oct 1;23(19):5981-5992. - Mateo J,* Carreira S*, Sandhu S*, Miranda S, Mossop H, Perez-Lopez R, et al DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. 2015. N Engl J Med 373(18):1697-1708.
- Goodall J*, Mateo J*, Yuan W, Mossop H, Porta N, Miranda S et al. Circulating Free DNA to Guide Prostate Cancer Treatment with PARP Inhibition. Cancer Discov. 2017 Sep;7(9):1006-1017
- Pritchard C*, Mateo J*, Walsh M*, de Sarkar N, Abida W, Beltran H, et al. Inherited DNA repair gene mutations in men with metastatic prostate cancer. N Engl J Med, 2016; 375(5)443-45.
- Robinson D, Van Allen EM, Wu Y-M, Schultz N, Lonigro R, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015;161(5):1215–28.
- Mateo J, Boysen G, Barbieri CE, Bryant HE, Castro E, Nelson PS, et al. DNA Repair in Prostate Cancer: Biology and Clinical Implications. European Urology. 2017 Mar;71(3):417-425.
- Seed, W. Yuan, J. Mateo, S. Carreira, C. Bertan, M. Lambros, G. Boysen, et al. Gene Copy Number Estimation From Targeted Next Generation Sequencing Of Prostate Cancer Biopsies: Analytic Validation and Clinical Qualification. Clinical Cancer Research, 2017 Oct 15;23(20):6070-6077
Todas las publicaciones
- Zurita AJ, Graf RP, Villacampa G, Raskina K, Sokol E, Jin D, Antonarakis ES, Li G, Huang RSP, Casanova-Salas I, Vivancos A, Carles J, Ross JS, Schrock AB, Oxnard GR, Mateo J. Genomic Biomarkers and Genome-Wide Loss-of-Heterozygosity Scores in Metastatic Prostate Cancer Following Progression on Androgen-Targeting Therapies. JCO Precis Oncol . 2022 Jul;6:e2200195.
- Cresta Morgado P, Mateo J. Clinical implications of homologous recombination repair mutations in prostate cancer. Prostate . 2022 Aug;82 Suppl 1:S45-S59.
- Westphalen CB, Fine AD, André F, Ganesan S, Heinemann V, Rouleau E, Turnbull C, Garcia Palacios L, Lopez JA, Sokol ES, Mateo J. Pan-cancer Analysis of Homologous Recombination Repair-associated Gene Alterations and Genome-wide Loss-of-Heterozygosity Score. Clin Cancer Res . 2022 Apr 1;28(7):1412-1421.
- Mateo J, Steuten L, Aftimos P, André F, Davies M, Garralda E, Geissler J, Husereau D, Martinez-Lopez I, Normanno N, Reis-Filho JS, Stefani S, Thomas DM, Westphalen CB, Voest E. Delivering precision oncology to patients with cancer. Nat Med . 2022 Apr;28(4):658-665
- Hussain M, Corcoran C, Sibilla C, Fizazi K, Saad F, Shore N, Sandhu S, Mateo J, Olmos D, Mehra N, Kolinsky MP, Roubaud G, Özgüroǧlu M, Matsubara N, Gedye C, Choi YD, Padua C, Kohlmann A, Huisden R, Elvin JA, Kang J, Adelman CA, Allen A, Poehlein C, de Bono J. Tumor Genomic Testing for >4,000 Men with Metastatic Castration-resistant Prostate Cancer in the Phase III Trial PROfound (Olaparib). Clin Cancer Res . 2022 Apr 14;28(8):1518-1530.
- Gillessen S, Bossi A, Davis ID, de Bono J, Fizazi K, James ND, Mottet N, Shore N, Small E, Smith M, Sweeney C, Tombal B, Antonarakis ES, Aparicio AM, Armstrong AJ, Attard G, Beer TM, Beltran H, Bjartell A, Blanchard P, Briganti A, Bristow RG, Bulbul M, Caffo O, Castellano D, Castro E, Cheng HH, Chi KN, Chowdhury S, Clarke CS, Clarke N, Daugaard G, De Santis M, Duran I, Eeles R, Efstathiou E, Efstathiou J, Ngozi Ekeke O, Evans CP, Fanti S, Feng FY, Fonteyne V, Fossati N, Frydenberg M, George D, Gleave M, Gravis G, Halabi S, Heinrich D, Herrmann K, Higano C, Hofman MS, Horvath LG, Hussain M, Alicja Jereczek-Fossa B, Jones R, Kanesvaran R, Kellokumpu-Lehtinen PL, Khauli RB, Klotz L, Kramer G, Leibowitz R, Logothetis CJ, Mahal BA, Maluf F, Mateo J, Matheson D, Mehra N, Merseburger A, Morgans AK, Morris MJ, Mrabti H, Mukherji D, Murphy DG, Murthy V, Nguyen PL, Oh WK, Ost P, O’Sullivan JM, Padhani AR, Pezaro C, Poon DMC, Pritchard CC, Rabah DM, Rathkopf D, Reiter RE, Rubin MA, Ryan CJ, Saad F, Pablo Sade J, Sartor OA, Scher HI, Sharifi N, Skoneczna I, Soule H, Spratt DE, Srinivas S, Sternberg CN, Steuber T, Suzuki H, Sydes MR, Taplin ME, Tilki D, Türkeri L, Turco F, Uemura H, Uemura H, Ürün Y, Vale CL, van Oort I, Vapiwala N, Walz J, Yamoah K, Ye D, Yu EY, Zapatero A, Zilli T, Omlin A. Management of Patients with Advanced Prostate Cancer. Part I: Intermediate-/High-risk and Locally Advanced Disease, Biochemical Relapse, and Side Effects of Hormonal Treatment: Report of the Advanced Prostate Cancer Consensus Conference 2022. Eur Urol . 2022 Dec 6:S0302-2838(22)02789-0.
- Mateo J, Beltran H. Moving Precision Oncology for Advanced Prostate Cancer from Theory to Practice. Eur Urol Focus . 2022 Aug 20:S2405-4569(22)00173-0.
- Mucci LA, Vinson J, Gold T, Gerke T, Filipenko J, Green RM, Anderson SG, Badal S, Bjartell A, Chi KN, Davis ID, Enting D, Fay AP, Lazarus J, Mateo J, McDermott R, Odedina FT, Olmos D, Omlin A, Popoola AA, Ragin C, Roberts R, Russnes KM, Waihenya C, Stopsack KH, Hyslop T, Villanti P, Kantoff PW, George DJ; IRONMAN Global Team. IRONMAN: A Novel International Registry of Men With Advanced Prostate Cancer. JCO Glob Oncol . 2022 Nov;8:e2200154.
- Graf RP, Fisher V, Mateo J, Gjoerup OV, Madison RW, Raskina K, Tukachinsky H, Creeden J, Cunningham R, Huang RSP, Mata DA, Ross JS, Oxnard GR, Venstrom JM, Zurita AJ. Predictive Genomic Biomarkers of Hormonal Therapy Versus Chemotherapy Benefit in Metastatic Castration-resistant Prostate Cancer. Eur Urol . 2022 Jan;81(1):37-47.
- Casanova-Salas I, Athie A, Boutros PC, Del Re M, Miyamoto DT, Pienta KJ, Posadas EM, Sowalsky AG, Stenzl A, Wyatt AW, Mateo J. Quantitative and Qualitative Analysis of Blood-based Liquid Biopsies to Inform Clinical Decision-making in Prostate Cancer. Eur Urol. 2021 Jun;79(6):762-771.
- Neeb A, Herranz N, Arce-Gallego S, Miranda S, Buroni L, Yuan W, Athie A, Casals T, Carmichael J, Rodrigues DN, Gurel B, Rescigno P, Rekowski J, Welti J, Riisnaes R, Gil V, Ning J, Wagner V, Casanova-Salas I, Cordoba S, Castro N, Fenor de la Maza MD, Seed G, Chandran K, Ferreira A, Figueiredo I, Bertan C, Bianchini D, Aversa C, Paschalis A, Gonzalez M, Morales- Barrera R, Suarez C, Carles J, Swain A, Sharp A, Gil J, Serra V, Lord C, Carreira S, Mateo J, de Bono JS. Advanced Prostate Cancer with ATM Loss: PARP and ATR Inhibitors. Eur Urol. 2021 Feb;79(2):200-211.
- Carreira S, Porta N, Arce-Gallego S, Seed G, Llop-Guevara A, Bianchini D, Rescigno P, Paschalis A, Bertan C, Baker C, Goodall J, Miranda S, Riisnaes R, Figueiredo I, Ferreira A, Pereira R, Crespo M, Gurel B, Nava Rodrigues D, Pettitt SJ, Yuan W, Serra V, Rekowski J, Lord CJ, Hall E, Mateo J, de Bono JS. Biomarkers Associating with PARP Inhibitor Benefit in Prostate Cancer in the TOPARP-B Trial. Cancer Discov. 2021 Nov;11(11):2812-2827.
- Westphalen B, Fine AD, Andre F, Ganesan S, Heinemann V, Rouleau E, Turnbull C, Garcia Palacios L, Lopez JA, Sokol ES, Mateo J. Pan-cancer Analysis of Homologous Recombination Repair-associated Gene Alterations and Genome-wide Loss of Heterozygosity Score. Clin Cancer Res. 2021 Nov 5:clincanres.2096.2021. Epub ahead of print.
- Mateo J, Seed G. Bertan C, Rescigno P, Dolling D, Figueiredo I, et al. (2019)Genomics of lethal prostate cancer at diagnosis and castration-resistance. J Clin Inv., ePub ahead of print, Dec.; DOI 10.1172/JCI132031.
- Mateo J, Porta N, Bianchini D, McGovern U, Elliot T, Jones R et al. (2019) Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncology, ePub ahead of print, Dec 2019; DOI: 10.1016/S1470-2045(19)30684-9.
- Athie A, Arce S, Gonzalez M, Morales R, Suarez C, Casals T, Hernandez G, Carles J, Mateo J. Targeting DNA Repair Defects for Precision Medicine in Prostate Cancer. Curr Onc Rep. 2019. 1007/s11912-019-0790-6
- Abida, Wassim; Cyrta, Joanna; Heller, Glenn; Prandi, Davide; Armenia, Joshua; Coleman, Ilsa; Cieslik, Marcin; Benelli, Matteo; Robinson, Dan; Van Allen, Eliezer M; Sboner, Andrea; Fedrizzi, Tarcisio; Mosquera, Juan Miguel; Robinson, Brian D; De Sarkar, Navonil; Kunju, Lakshmi P; Tomlins, Scott; Wu, Yi Mi; Nava Rodrigues, Daniel; Loda, Massimo; Gopalan, Anuradha; Reuter, Victor E; Pritchard, Colin C; MATEO, JOAQUIN; Bianchini, Diletta; Miranda, Susana; Carreira, Suzanne; Rescigno, Pasquale; Filipenko, Julie; Vinson, Jacob; Montgomery, Robert B; Beltran, Himisha; Heath, Elisabeth I; Scher, Howard I; Kantoff, Philip W; Taplin, Mary-Ellen; Schultz, Nikolaus; deBono, Johann S; Demichelis, Francesca; Nelson, Peter S; Rubin, Mark A; Chinnaiyan, Arul M; Sawyers, Charles L. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Nat Acad Sci. 2019. 1073/pnas.1902651116
- Zafeiriou, Zafeiris; Bianchini, Diletta; Chandler, Robert; Rescigno, Pasquale; Yuan, Wei; Carreira, Suzanne; Barrero, Maialen; Petremolo, Antonella; Miranda, Susana; Riisnaes, Ruth; Rodrigues, Daniel Nava; Gurel, Bora; Sumanasuriya, Semini; Paschalis, Alec; Sharp, Adam; MATEO, JOAQUIN; Tunariu, Nina; Chinnaiyan, Arul M; Pritchard, Colin C; Kelly, Kevin; de Bono, Johann S. Genomic Analysis of Three Metastatic Prostate Cancer Patients with Exceptional Responses to Carboplatin Indicating Different Types of DNA Repair Deficiency. European Urology, 2019. 10.1016/j.eururo.2018.09.048
- Nava Rodrigues, Daniel; Casiraghi, Nicola; Romanel, Alessandro; Crespo, Mateus; Miranda, Susana; Rescigno, Pasquale; Figueiredo, Ines; Riisnaes, Ruth; Carreira, Suzanne; Sumanasuriya, Semini; Gasperini, Paola; Sharp, Adam; MATEO, JOAQUIN; Makay, Alan; McNair, Christopher; Schiewer, Matthew; Knudsen, Karen; Boysen, Gunther; Demichelis, Francesca; de Bono, Johann S. Clin Cancer Res, 2019. 10.1158/1078-0432.CCR-18-2068
- MATEO, JOAQUIN; Fizazi, Karim; Gillessen, Silke; Heidenreich, Axel; PEREZ LOPEZ, RAQUEL; Oyen, Wim J G; Shore, Neal; Smith, Matthew; Sweeney, Christopher; Tombal, Bertrand; Tomlins, Scott A; de Bono, Johann S. Managing Nonmetastatic Castration-resistant Prostate Cancer. European Urology, 2019. 10.1016/j.eururo.2018.07.035
- MATEO, JOAQUIN; Lord, C J; SERRA, VIOLETA; Tutt, A; BALMAÑA, JUDITH; CASTROVIEJO, CRISTINA; CRUZ, CRISTINA; OAKNIN, ANA; Kaye, S B; de Bono, J S. A decade of clinical development of PARP inhibitors in perspective. Ann Onc, 2019. 10.1093/annonc/mdz192
- Sharp, Adam; Welti, Jon C; Lambros, Maryou B K; Dolling, David; Rodrigues, Daniel Nava; Pope, Lorna; Aversa, Caterina; Figueiredo, Ines; Fraser, Jennifer; Ahmad, Zai; Lu, Changxue; Rescigno, Pasquale; Kolinsky, Michael; Bertan, Claudia; Seed, George; Riisnaes, Ruth; Miranda, Susana; Crespo, Mateus; Pereira, Rita; Ferreira, Ana; Fowler, Gemma; Ebbs, Berni; Flohr, Penny; Neeb, Antje; Bianchini, Diletta; Petremolo, Antonella; Sumanasuriya, Semini; Paschalis, Alec; MATEO, JOAQUIN; Tunariu, Nina; Yuan, Wei; Carreira, Suzanne; Plymate, Stephen R; Luo, Jun; de Bono, Johann S. Clinical Utility of Circulating Tumour Cell Androgen Receptor Splice Variant-7 Status in Metastatic Castration-resistant Prostate Cancer. European Urology. 10.1016/j.eururo.2019.04.006
- Mateo J, Cheng HH, Beltran H, Dolling D, Xu W, Pritchard C, et al. Clinical outcome of prostate cancer patients with germline DNA repair mutations: follow-up from an international study. Eur Urol. 2018 doi:10.1016/j.euro.2018.01.010
- Nava-Rodrigues D, Rescigno P, Liu D, Yuan W, Carreira S, Lambros M, et al. Immunogenomic analyses associate immunological alterations with mismatch repair defects in prostate cancer, J Clin Inv. 2018; 128-11, pp.5185-5185.
- Mateo J, Chakravarty D, Dienstmann R, Jezdic S, Gonzalez-Perez A, Lopez-Bigas N, et al. A framework to rank genomic alterations as targets for cancer precision medicine: The ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol., August 2018, 29-9: 1895-1902.
Boysen G, Nava Rodrigues D, Rescigno P, Seed G, Dolling DI, Riisnaes R, Crespo M, et al. SPOP mutated/ CHD1 deleted lethal prostate cancer and abiraterone sensitivity. Clin Can Res. 2018; 24-22: 5585-5593. - Mateo J, Carles J. 2018. Towards a New Classification for Metastatic Prostate Cancer, Eur Urol.
- Lorente, D, Olmos D, Mateo J et al. 2018. Circulating tumour cell increase as a biomarker of disease progression in metastatic castration-resistant prostate cancer patients with low baseline CTC counts. Ann Oncol. 29-7, pp.1554-1560.
- Joaquin Mateo , Gopinath Ganji , Charlotte Lemech , Howard A. Burris , Sae-Won Han , Karen Swales , Shaun Decordova , Maurice P DeYoung , Deborah A Smith , Shanker Kalyana-Sundaram , Jiuhua Wu , Monica Motwani , Rakesh Kumar , Jerry M Tolson , Sun Young Rha , Hyun Cheol Chung , Joseph Paul Eder , Sunil Sharma, Yung-Jue Bang , Jeffrey R. Infante ,Li Yan, Johann de Bono, Hendrik-Tobias Arkenau. A first-time-in-human study of GSK2636771, a phosphoinositide 3 kinase beta-selective inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2017 Oct 1;23(19):5981-5992.
- Seed G, Yuan W, Mateo J, Carreira S, Bertan C, Lambros M, Boysen G, Ferraldeschi R, Miranda S, Figueiredo I, Riisnaes R, Crespo M, Rodrigues DN, Talevich E, Robinson DR, Kunju LP, Wu YM, Lonigro R, Sandhu S, Chinnayan A, de Bono JS. Gene Copy Number Estimation from Targeted Next-Generation Sequencing of Prostate Cancer Biopsies: Analytic Validation and Clinical Qualification. Clin Cancer Res. 2017 Oct 15;23(20):6070-6077.
- Goodall J*, Mateo J*, Yuan W, Mossop H, Porta N, Miranda S, Perez-Lopez R, Dolling D, Robinson DR, Sandhu S, Fowler G, Ebbs B, Flohr P, Seed G, Nava Rodrigues D, Boysen G, Bertan C, Atkin M, Clarke M, Crespo M, Figueiredo I, Riisnaes R, Sumanasuriya S, Rescigno P, Zafeiriou Z, Sharp A, Tunariu N, Bianchini D, Gillman A, Lord CJ, Hall E, Chinnaiyan AM, Carreira S, de Bono JS. Circulating Free DNA to Guide Prostate Cancer Treatment with PARP Inhibition. Cancer Discovery. 2017 Apr 27 [Epub ahead of print] DOI: 10.1158/2159-8290.CD-17-0261
- Massard C, Mateo J, Loriot Y, Pezaro C, Albiges L, Mehra N, Varga A, Bianchini D, Ryan CJ, Petrylak DP, Attard G, Shen L, Fizazi K, de Bono J. : Phase I/II trial of cabazitaxel plus abiraterone in patients with metastatic castration-resistant prostate cancer (mCRPC) progressing after docetaxel and abiraterone. Annals of Oncology. 2017 Jan 1;28(1):90-95
- Pritchard C*, Mateo J*, Walsh M*, de Sarkar N, Abida W, Beltran H, Garofalo A, Gulati R, Carreira S, Eeles R, Elemento O, Rubin M, Robinson D, Lonigro R, Hussein M, Chinnaiyan A, Vinson J, Filipenko J, Garraway L, Taplin ME, Aldubayan S, Han C, Beightol M, Morissey C, Nghiem B, Cheng H, Montgomery B, Walsh T, Casadei S, Vijai J, Scher H, Sawyers C, Schultz N, Kantoff P, Solit D, Robson M, Van Allen E, Offit K, de Bono JS, Nelson P. (on behalf of the SU2C-PCF Prostate Cancer Dream Team] Inherited DNA repair gene mutations in men with metastatic prostate cancer. N Engl J Med, 2016; 375(5)443-45.
- Mateo J,* Carreira S*, Sandhu S*, Miranda S, Mossop H, Perez-Lopez R, Nava Rodrigues D, Robinson D, Omlin A, Tunariu N, Boysen G, Porta N, Flohr P, Gillman A, Figueiredo I, Paulding C, Seed G, Jain S, Ralph C, Protheroe A, Hussain S, Jones R, Elliott T, McGovern U, Bianchini D, Goodall J, Zafeiriou Z, Williamson CT, Ferraldeschi R, Riisnaes R, Ebbs B, Fowler G, Roda D, Yuan W, Wu YM, Cao X, Brough R, Pemberton H, A’Hern R, Swain A, Kunju LP, Eeles R, Attard G, Lord CJ, Ashworth A, Rubin MA, Knudsen KE, Feng FY, Chinnaiyan AM, Hall E, de Bono JS. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. 2015. N Engl J Med 373(18):1697-1708.
- Robinson D, Van Allen EM, Wu Y-M, Schultz N, Lonigro R, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC, Attard G, Beltran H, Abida W, Bradley RK, Vinson J, Cao X, Vats P, Kunju LP, Hussain M, Feng F, Tomlins SA, Conney KA, Smith DC, Brennan C, Siddiqui J, Mehra R, Chen Y, Rathkopf DE, Morris MJ, Solomon SB, Durack JC, Reuter VE, Gopalan A, Gao J, Loda M, Lis RT, Bowden M, Balk SP, Gaviola G, Sougnez C, Gupta M, Yu, EY, Mostaghel EA, Cheng HH, Mulcahy H, True L, Plymate SR, Dyinge H, Ferraldeschi R, Flohr P, Miranda S, Zafeiriou Z, Tunariu N, Mateo J, Perez-Lopez R, Demichelis F, Robinson B, Schiffman M, Nanus DM, Tagawa ST, Sigaras A, Eng KW, Elemento O, Sboner A, Heath EI, Scher HI, Pienta KJ, Kantoff P, de Bono JS, Rubin MA, Nelson PA, Garraway LA, Sawyers CL, Chinnaiyan AM. Integrative clinical genomics of advanced prostate cancer. Cell 2015;161(5):1215–28.
- Perez-Lopez R, Mateo J, Mossop H, Blackledge MD, Collins DJ, Rata M, Morgan VA, Macdonald A, Sandhu S, Lorente D, Rescigno P, Zafeiriou Z, Bianchini D, Porta N, Hall E, Leach MO, de Bono JS, Koh DM, Tunariu N. Diffusion-weighted Imaging as a Treatment Response Biomarker for Evaluating Bone Metastases in Prostate Cancer: A Pilot Study. Radiology. 2017 Apr;283(1):168-177
- Perez-Lopez R, Lorente D, Blackledge MD, Collins DJ, Mateo J, Bianchini D, Omlin A, Zivi A, Leach MO, de Bono JS, Koh DM, Tunariu N. Volume of Bone Metastasis Assessed with Whole-Body Diffusion-weighted Imaging Is Associated with Overall Survival in Metastatic Castration-resistant Prostate Cancer. Radiology 2016; 280(1):151-160.
- Ferraldeschi R, Nava D, Riisnaes R, Miranda S, Figueiredo I, Rescigno P, Ravi P, Pezaro C, Omlin A, Lorente D, Zafeiriou Z, Mateo J, Altavilla A, Sideris S, Bianchini D, Girst E, Thway K, Perez-Lopez R, Tunariu N, Parker C, Dearnaley D, Reid A, Attard G, de Bono JS. PTEN protein loss and clinical outcome from castration-resistant prostate cancer treated with abiraterone acetate. European Urology 2015;67(4):795–802.
- Mateo J, Olmos D, Dumez H, Poondru S, Samberg N, Barr S, Van Tornout JM, Jie F, Sandhu S, Tan DS, Moreno V, LoRusso PM, Kaye SB, Schoffski P. A first-in man, dose-finding study of the mTORC1/mTORC2 inhibitor OSI-027 in patients with advanced solid malignancies. Br J Cancer 2016, 114, 889-896
- Mateo J, Moreno V, Gupta A, Kaye SB, Dean E, Middleton MR, Friedlander M, Gourley C, Plummer R, Rustin G, Sessa C, Leunen K, Ledermann J, Swaisland H, Fielding A, Bannister W, Nicum S, Molife LR. An adaptive study to determine the optimal dose of the tablet formulation of the PARP inhibitor Olaparib. Target Oncol. 2016 Jun;11(3):401-15
- McDaniel AS, Ferraldeschi R, Krupa R, Landers M, Graf R, Louw J, Jendrisak A, Bales N, Marrinucci D, Zafeiriou Z, Flohr P, Sideris S, Crespo M, Figueiredo I, Mateo J, de Bono JS, Dittamore R, Tomlins SA, Attard G. Phenotypic diversity of circulating tumour cells in patients with metastatic castration-resistant prostate cancer. BJU Int. 2016 Aug (EPub)
- Rescigno P, Lorente D, Bianchini D, Ferraldeschi R, Kolinsky MP, Sideris S, Zafeiriou Z, Sumanasuriya S, Smith AD, Mehra N, Jayaram A, Perez-Lopez R, Mateo J, Parker C, Dearnaley DP, Tunariu N, Reid A, Attard G, de Bono JS. Prostate-specific Antigen Decline After 4 Weeks of Treatment with Abiraterone Acetate and Overall Survival in Patients with Metastatic Castration-resistant Prostate Cancer. European Urology. 2016 Nov;70(5):724-731.
- Lorente D, Olmos D, Mateo J, Bianchini D, Seed G, Fleisher M, Danila DC, Flohr P, Crespo M, Figueiredo I, Miranda S, Baeten K, Molina A, Kheoh T, McCormack R, Terstappen LW, Scher HI, de Bono JS. Decline in Circulating Tumor Cell Count and Treatment Outcome in Advanced Prostate Cancer. European Urology. 2016; 70*6)985-992.
- Lorente D, Omlin A, Zafeiriou Z, Nava-Rodrigues D, Pérez-López R, Pezaro C, Mehra N, Sheridan E, Figueiredo I, Riisnaes R, Miranda S, Crespo M, Flohr P, Mateo J, Altavilla A, Ferraldeschi R, Bianchini D, Attard G, Tunariu N, de Bono J. Castration-Resistant Prostate Cancer Tissue Acquisition From Bone Metastases for Molecular Analyses. Clin Genitourin Cancer. 2016 Dec;14(6):485-493
- Frenel J-S, Carreira S, Goodall J, Roda-Perez D, Perez-Lopez R, Tunariu N, Riisnaes R, Miranda S, Figueiredo I, Nava Rodrigues D, Smith A, Leux C, Garcia-Murillas I, Ferraldeschi R, Lorente D, Mateo J, Ong M, Yap TA, Banerji U, Gasi D, Turner N, Attard G, de Bono JS. Serial Next Generation Sequencing of Circulating Cell Free DNA Evaluating Tumour Clone Response To Molecularly Targeted Drug Administration. Clin Cancer Res 2015; 21(20):4586-96.
- Lorente D, Mateo J, Templeton AJ, Zafeiriou Z, Bianchini D, Ferraldeschi R, Bahl A, Shen L, Su Z, Sartor O, de Bono JS. Baseline neutrophil-lymphocyte ratio (NLR) is associated with survival and response to treatment with second-line chemotherapy for advanced prostate cancer independent of baseline steroid use. Ann Oncol. 2015 Apr;26(4):750-5
- Ferraldeschi R, Nava Rodrigues D, Riisnaes R, Miranda S, Figueiredo I, Rescigno P, Ravi P, Pezaro C, Omlin A, Lorente D, Zafeiriou Z, Mateo J, Altavilla A, Sideris S, Bianchini D, Grist E, Thway K, Perez Lopez R, Tunariu N, Parker C, Dearnaley D, Reid A, Attard G, de Bono J. PTEN protein loss and clinical outcome from castration-resistant prostate cancer treated with abiraterone acetate. Eur Urol. 2015 Apr;67(4):795-802
- Wilbaux M, Tod M, De Bono J, Lorente D, Mateo J, Freyer G, You B, Hénin E. A Joint Model for the Kinetics of CTC Count and PSA Concentration During Treatment in Metastatic Castration-Resistant Prostate Cancer. CPT Pharmacometrics Syst Pharmacol. 2015 May;4(5):277-85
- Mateo J, Berlin J, de Bono JS, Cohen RB, Keedy V, Mugundu G, Zhang L, Abbattista A, Davis C, Gallo Stampino C, Borghaei H. A first-in-human study of the anti-α5β1 integrin monoclonal antibody PF-04605412 administered intravenously to patients with advanced solid tumors. Cancer Chemother Pharmacol 2014, 74(5):1039-46.
- Ong M, Carreira S, Goodall J, Mateo J, Figueiredo I, Rodrigues DN, Perkins G, Seed G, Yap TA, Attard G, de Bono JS. Validation and utilisation of high-coverage next-generation sequencing to deliver the pharmacological audit trail. Br J Cancer 2014;111(5):828-36.
- Ravi P, Mateo J, Lorente D, Zafeiriou Z, Altavilla A, Ferraldeschi R, Sideris S, Grist E, Smith A, Wong S, Bianchinni D, Attard G, de Bono JS. External Validation of a Prognostic Model Predicting Overall Survival in Metastatic Castrate-resistant Prostate Cancer Patients Treated with Abiraterone. European Urology 2014;66(1):8–11.
- Barber LJ, Sandhu S, Chen L, Campbell J, Kozarewa I, Fenwick K, Assiotis I, Rodrigues DN, Reis Filho JS, Moreno V, Mateo J, Molife LR, De Bono J, Kaye S, Lord CJ, Ashworth A. Secondary mutations in BRCA2 associated with clinical resistance to a PARP inhibitor. J Pathol. 2013 Feb;229(3):422-9.
- Mateo J, Carreira S, de Bono JS. Acquiring evidence for precision prostate cancer care. Annals Oncology. 2017 Apr 13. (Epub ahead of print)
- Mateo J, Sharp A, de Bono JS. Investigating Genomic Aberrations of the Androgen Receptor: Moving Closer to More Precise Prostate Cancer Care? European Urology. 2017 Feb 20.
- Mateo J, Boysen G, Barbieri CE, Bryant HE, Castro E, Nelson PS, Olmos D, Pritchard CC, Rubin MA, de Bono JS. DNA Repair in Prostate Cancer: Biology and Clinical Implications. European Urology. 2017 Mar;71(3):417-425.
- Lorente D, Mateo J, Perez-Lopez R, de Bono JS, Attard G. Sequencing agents in metastatic prostate cancer. Lancet Oncology 2015 Jun;16(6):e279-92.
- Lorente D, Mateo J, Zafeiriou Z, Smith AD, Sandhu S, Ferraldeschi R, de Bono JS. Switching and withdrawing hormonal agents for castration-resistant prostate cancer. Nat Rev Urol. 2015 Jan;12(1):37-47.
- Mateo J, Gerlinger M, Rodrigues DN, de Bono JS. The promise of circulating tumor cell analysis in cancer management Genome Biol. 2014 Aug 30;15(8):448.
- Mateo J, Ong M, Tan DS, Gonzalez MA, de Bono JS. Appraising iniparib, the PARP inhibitor that never was–what must we learn? Nat Rev Clin Oncol. 2013 Dec;10(12):688-696.
- Clinical Qualification of DNA Repair Defects as Biomarkers in Metastatic Prostate Cancer Using Integrated Genomics and Tissue-Based Functional Assays.
Grantor: US Department of Defense Congressionally-Directed Medical Research Program. - Co-targeting androgen receptor signalling and DNA damage repair for precision therapy in advanced prostate.
Grantor: European Commission H2020 Program. - Novel approaches to liquid biopsy in prostate cancer to inform precision medicine.
Grantor: FERO Foundation. - Leveraging the AR-DDR interaction in de-novo metastatic prostate cancer towards precision combination therapies with PARP inhibitors.
Grantor: AECC (Fundación Asociación Española contra el Cáncer). - Integrated analysis of blood circulating tumor signatures to monitor genomic evolution and therapy responses in advanced Prostate Cancer.
Grantor: ”la Caixa” - Exploiting senescence in a two-step approach to treat advanced Prostate Cancer.
Grantor: Instituto de Salud Carlos III (ISCIII). - Tumoral senescence induced by anti-cancer therapies constitutes a novel prognostic biomarker and a therapeutic target.
Grantor: AECC (Fundación Asociación Española contra el Cáncer). - Prostate Cancer Genomic Evolution and Signatures of DNA Damage Repair Deficiencies.
Grantor: CRIS Cancer Foundation. - Title: Development of NT1, a potent and selective best-in-class drug targeting the Androgen Receptor disordered domain for the treatment of Castration Resistant Prostate Cancer . Grantor: Agencia Estatal de Investigación – Ministerio de Ciencia e Innovación. Ref: CPP2022-009651. Duration: 01/10/2023 – 30/09/2026. Coordinator: Nuage Therapeutics, S.L.. Other Partners: Fundació Institut de Recerca Biomédica (Barcelona). PI VHIO: Joaquín Mateo.


















