VHIO’s Cancer Genomics Group serves as a Core Technology laboratory. We are also dedicated to translational research as well as the development of novel genomic tests.
Our group provides cutting-edge applications in cancer genomics through state-of-the-art technologies and we develop new, fully validated tests that are used in the clinical research setting. Our lab is equipped with an n-Counter (Nanostring) platform, two digital PCR platforms (BEAMing Sysmex and ddPCR, BIO-RAD) and five NextGen Sequencers; MiSeq, NextSeq, HiSeq2500 and NovaSeq6000 from Illumina, and a MinION from Oxford Nanopore Technologies.
Our lab completed the technology transfer of the Food and Drug Administration (FDA)-approved Guardant360® CDx liquid biopsy test for comprehensive genomic profiling. With this test, VHIO360, our Institute is the first cancer research center in Europe to have a laboratory equipped with this cutting-edge platform. Aimed at overcoming the limitations and challenges of tissue biopsies, this technology provides complete genomic results in all solid tumors from a simple blood draw in seven days.
Molecular Prescreening at VHIO is co-led by our group’s Principal Investigator, Ana Vivancos, alongside Paolo Nuciforo, Elena Garralda, and Rodrigo Dienstmann, Principal Investigators of our Molecular Oncology, Early Clinical Drug Development, and Oncology Data Science – OdysSey Groups, respectively. Supported through our institutional Advanced Molecular Diagnostics Program (DIAMAV), powered by the Fundación FERO, we perform molecular profiling in over 1100 patients each year as potential candidates for enrollment in our phase I clinical trials led by VHIO’s Research Unit for Molecular Therapy of Cancer (UITM) – CaixaResearch, directed by Elena Garralda. Patients’ suitability for inclusion in any given clinical study is assessed based on their respective genomic profile and pathologic features.
We have developed and routinely implemented several tests for this program. Two are based on NGS: an Amplicon-seq approach to sequence 67 genes as well as a 450-gene capture panel (Illumina). We use nCounter (Nanostring) for our RNA- based gene fusion panel, with the capacity of detecting over 100 recurrent gene fusions (also enabling us to assess gene expression patterns in tumors). As a reflection of our dedication to excellence and quality in the services that we provide, we have attained ISO 15189 flexible accreditation for both our Amplicon-seq testing and large 450-gene capture panel.
Research activities focus on developing novel multiplexed tests that are optimized to FFPE-derived nucleic acids. Once developed, they are validated and used in both clinical and translational research. We are also involved in several translational research projects including the identification of mechanisms of resistance to targeted therapies, as well as predictive biomarkers for immunotherapies. Based on Nanostring and RNA-seq technologies for the detection of an immune signature, we use the VIGex tool. Our group is particularly interested in liquid biopsy and RNA-based analysis of tumors for microenvironment profiling.
- Develop and implement improved strategies for routine patient prescreening with a large pancancer panel in a setting of excellence.
- Provide cutting-edge applications in cancer genomics through the use of novel technologies and protocol development.
- Prioritize translational projects and partnerships that reinforce VHIO’s renowned excellence in oncology.
- Implement the Guardant 360® DX test in liquid biopsy as the first laboratory in Europe to perform the assay from Guardant Health.
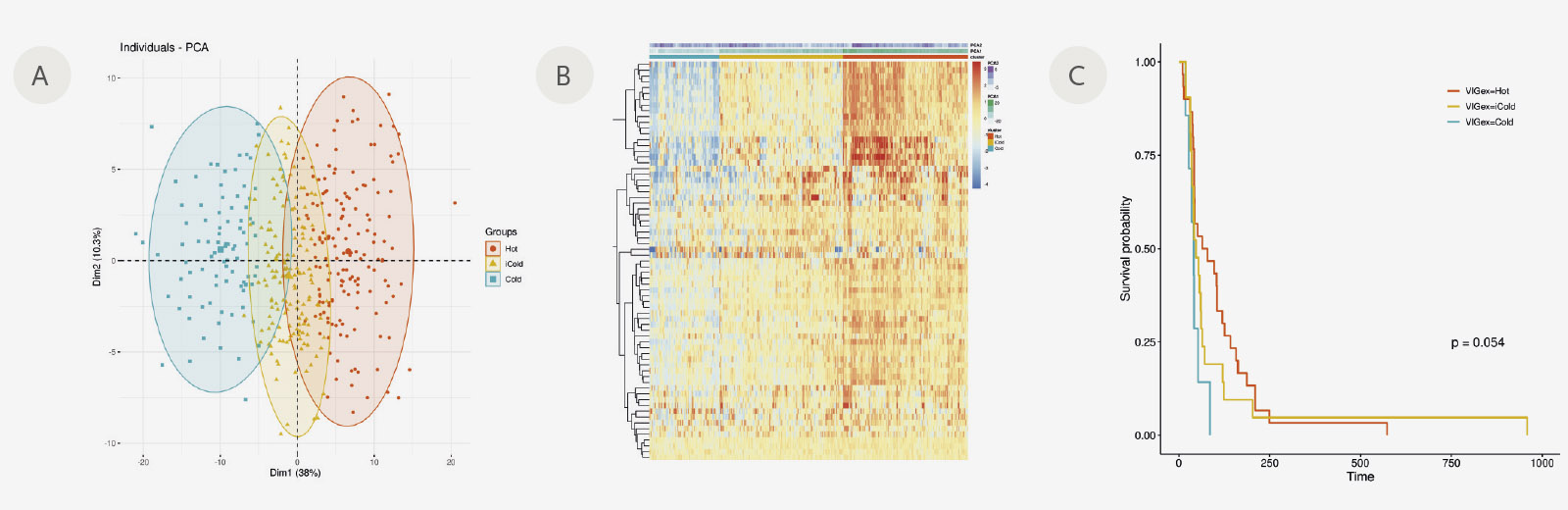
Figure: VIGex classification of 398 cancer metastatic samples according to nCounter (Nanostring) gene expression (69 immuno-related genes). Gene expression values were normalized to the geometric mean expression of 19 housekeeping genes, then log2-transformed and centred around mean. A) PCA showing the 3 clusters identified with PAM (partitioning around medoids) method (Hot, iCold and Cold). B) Heatmap showing relative gene expression and PCA values of the 69 immuno-related genes within Hot, iCold and Cold groups. C) Kaplan-Meier plot showing time to progression of the Hot, iCold and Cold groups of an independent cohort of 58 samples.

Group Leader
Ana Vivancos
Laboratory manager
Judit Matito
Project manager
Ester Castillo
Specialized Technicians
Deborah G. Lo Giacco
Zighereda Ogbah
Cecilia García
Giuseppe Buono
Vanessa Bach,
Agatha Martín
Bioinformaticians
Francisco Fuster
Marina Gómez
Maria Vila
Research Support Technician
Inmaculada Martos
Most relevant scientific publications
- Élez E, Mulet-Margalef N, Sanso M, Ruiz-Pace F, Mancuso FM, Comas R, Ros J, Argilés G, Martini G, Sanz-Garcia E, Baraibar I, Salvà F, Noguerido A, Cuadra-Urteaga JL, Fasani R, Garcia A, Jimenez J, Aguilar S, Landolfi S, Hernández-Losa J, Braña I, Nuciforo P, Dienstmann R, Tabernero J, Salazar R, Vivancos A. A Comprehensive Biomarker Analysis of Microsatellite Unstable/Mismatch Repair Deficient Colorectal Cancer Cohort Treated with Immunotherapy. Int J Mol Sci. 2022 Dec 21;24(1):118
- Vivancos A, Tabernero J. Circulating tumor DNA as a novel prognostic indicator. Nat Med. 2022 Nov;28(11):2255-2256.
- Boix O, Martinez M, Vidal S, Giménez-Alejandre M, Palenzuela L, Lorenzo-Sanz L, Quevedo L, Moscoso O, Ruiz-Orera J, Ximénez-Embún P, Ciriaco N, Nuciforo P, Stephan-Otto Attolini C, Albà MM, Muñoz J, Tian TV, Varela I, Vivancos A, Ramón Y Cajal S, Muñoz P, Rivas C, Abad M. pTINCR microprotein promotes epithelial differentiation and suppresses tumor growth through CDC42 SUMOylation and activation. Nat Commun. 2022 Nov 11;13(1):6840.
- Garcia-Casado Z, Oaknin A, Mendiola M, Alkorta-Aranburu G, Antunez-Lopez JR, Moreno-Bueno G, Palacios J, Yubero A, Marquez R, Gallego A, Sanchez-Heras AB, Lopez-Guerrero JA, Perez-Segura C, Barretina-Ginesta P, Alarcon J, Gaba L, Marquez A, Matito J, Cueva J, Palacio I, Iglesias M, Arcusa A, Sanchez-Lorenzo L, Guerra-Alia E, Romero I, Vivancos A. Laboratory Cross-Comparison and Ring Test Trial for Tumor BRCA Testing in a Multicenter Epithelial Ovarian Cancer Series: The BORNEO GEICO 60-0 Study. J Pers Med. 2022 Nov 4;12(11):1842.
- Capdevila J, Mayor R, Mancuso FM, Iglesias C, Caratú G, Matos I, Zafón C, Hernando J, Petit A, Nuciforo P, Cameselle-Teijeiro JM, Álvarez CV, Recio JA, Tabernero J, Matias-Guiu X, Vivancos A, Seoane J. Early evolutionary divergence between papillary and anaplastic thyroid cancers. Ann Oncol.2019 Nov 1;30(11):1843.
- Vivancos A, Élez E, Salazar R.Circulating cell-free DNA as predictor of treatment failure after neoadjuvant chemoradiotherapy before surgery in patients with locally advanced rectal cancer: is it ready for primetime? Ann Oncol.29: 532-534.
- Capdevila J,Mayor R; Mancuso FF, Iglesias C; Caratù G, Matos I, Zafón C, Hernando J, Petit A, Nuciforo P, Cameselle-Teijeiro JM, Álvarez C, Recio JA, Tabernero J, Matias-Guiu X, Vivancos A, Seoane J.Early evolutionary divergence between papillary and anaplastic thyroid cancers. Ann Oncol.29: 1454-1460.
- Cedrés S, Felip E, Cruz C, Martinez de Castro A, Pardo N, Navarro A, Martinez-Marti A, Remon J, Zeron-Medina J, Balmaña J, Llop-Guevara A, Miquel JM, Sansano I, Nuciforo P,Mancuso F, Serra V, Vivancos A. Activity of HSP90 Inhibiton in a Metastatic Lung Cancer Patient With a Germline BRCA1 Mutation. J Natl Cancer Inst.110: 914-917.
- Puig I, Tenbaum SP, Chicote I, Arqués O,Martínez-Quintanilla J, Cuesta-Borrás E, Ramírez L, Gonzalo P, Soto A, Aguilar S, Eguizabal C, Caratù G, Prat A, Argilés G, Landolfi S, Casanovas O, Serra V, Villanueva A, Arroyo AG, Terracciano L, Nuciforo P,Seoane J,Recio JA, Vivancos A,Dienstmann R,Tabernero J,Palmer HG.TET2 controls chemoresistant slow-cycling cancer cell survival and tumor recurrence. J Clin Invest.128: 3887-3905.
- Cruz C, Castroviejo-Bermejo M, Gutiérrez-Enríquez S,Llop-Guevara A, Ibrahim YH, Gris-Oliver A, Bonache S, Morancho B, Bruna A, Rueda OM, Lai Z, Polanska UM, Jones GN; Kristel P, de Bustos L, Guzman M,Rodriguez O, Grueso J, Montalban G, Caratú G,Mancuso F, Fasani R, Jiménez J, Howat WJ, Dougherty B, Vivancos A, Nuciforo P, Serres-Créixams X, Rubio IT, Oaknin A, Cadogan E, Barrett JC, Caldas C, Baselga J, Saura C, Cortés J, Arribas J, Jonkers J, Díez O; O’Connor MJ, Balmaña J,Serra V. RAD51 foci as a functional biomarker of homologous recombination repair and PARP inhibitor resistance in germline BRCA-mutated breast cancer. Ann Oncol.29: 1203-1210. IF: 13,926
- Martínez-Ricarte F, Mayor R, Martínez-Sáez E, Rubio-Pérez C, Pineda E, Cordero E, Cicuéndez M, Poca MA, Lopez-Bigas N, Ramón Y Cajal S, Vieito M, Carles J, Tabernero J,Vivancos A, Gallego S, Graus F, Sahuquillo J, Seoane J. Molecular Diagnosis of Diffuse Gliomas through Sequencing of Cell-Free Circulating Tumor DNA from Cerebrospinal Fluid.Clin Cancer Res.24: 2812-2819.
All publications
- Élez E, Mulet-Margalef N, Sanso M, Ruiz-Pace F, Mancuso FM, Comas R, Ros J, Argilés G, Martini G, Sanz-Garcia E, Baraibar I, Salvà F, Noguerido A, Cuadra-Urteaga JL, Fasani R, Garcia A, Jimenez J, Aguilar S, Landolfi S, Hernández-Losa J, Braña I, Nuciforo P, Dienstmann R, Tabernero J, Salazar R, Vivancos A. A Comprehensive Biomarker Analysis of Microsatellite Unstable/Mismatch Repair Deficient Colorectal Cancer Cohort Treated with Immunotherapy. Int J Mol Sci. 2022 Dec 21;24(1):118. doi: 10.3390/ijms24010118. PMID: 36613564; PMCID: PMC9820517.
- Palomero J, Panisello C, Lozano-Rabella M, Tirtakasuma R, Díaz-Gómez J, Grases D, Pasamar H, Arregui L, Dorca Duch E, Guerra Fernández E, Vivancos A, de Andrea CE, Melero I, Ponce J, Vidal A, Piulats JM, Matias-Guiu X, Gros A. Biomarkers of tumor-reactive CD4<sup>+</sup> and CD8<sup>+</sup> TILs associate with improved prognosis in endometrial cancer. J Immunother Cancer. 2022 Dec;10(12):e005443. doi: 10.1136/jitc-2022-005443. PMID: 36581331; PMCID: PMC9806064.
- Garcia-Casado Z, Oaknin A, Mendiola M, Alkorta-Aranburu G, Antunez-Lopez JR, Moreno-Bueno G, Palacios J, Yubero A, Marquez R, Gallego A, Sanchez-Heras AB, Lopez-Guerrero JA, Perez-Segura C, Barretina-Ginesta P, Alarcon J, Gaba L, Marquez A, Matito J, Cueva J, Palacio I, Iglesias M, Arcusa A, Sanchez-Lorenzo L, Guerra-Alia E, Romero I, Vivancos A. Laboratory Cross-Comparison and Ring Test Trial for Tumor <i>BRCA</i> Testing in a Multicenter Epithelial Ovarian Cancer Series: The BORNEO GEICO 60-0 Study. J Pers Med. 2022 Nov 4;12(11):1842. doi: 10.3390/jpm12111842. PMID: 36579549; PMCID: PMC9698073.
- Guarneri V, Bras-Maristany F, Dieci MV, Griguolo G, Par L, Mar Ín-Aguilera M, Miglietta F, Bottosso M, Giorgi CA, Blasco P, Castillo O, Galv N P, Vivancos A, Villagrasa P, Parker JS, Perou CM, Conte P, Prat A. HER2DX genomic test in HER2-positive/hormone receptor-positive breast cancer treated with neoadjuvant trastuzumab and pertuzumab: A correlative analysis from the PerELISA trial. EBioMedicine. 2022 Nov;85:104320. doi: 10.1016/j.ebiom.2022.104320. Epub 2022 Oct 29. PMID: 36374768; PMCID: PMC9626543.
- Devis-Jauregui L, Vidal A, Plata-Peña L, Santacana M, García-Mulero S, Bonifaci N, Noguera-Delgado E, Ruiz N, Gil M, Dorca E, Llobet FJ, Coll-Iglesias L, Gassner K, Martinez-Iniesta M, Rodriguez-Barrueco R, Barahona M, Marti L, Viñals F, Ponce J, Sanz-Pamplona R, Piulats JM, Vivancos A, Matias-Guiu X, Villanueva A, Llobet-Navas D. Generation and Integrated Analysis of Advanced Patient-Derived Orthoxenograft Models (PDOX) for the Rational Assessment of Targeted Therapies in Endometrial Cancer. Adv Sci (Weinh). 2022 Nov 14;10(1):e2204211. doi: 10.1002/advs.202204211. Epub ahead of print. PMID: 36373729; PMCID: PMC9811454.
- Boix O, Martinez M, Vidal S, Giménez-Alejandre M, Palenzuela L, Lorenzo-Sanz L, Quevedo L, Moscoso O, Ruiz-Orera J, Ximénez-Embún P, Ciriaco N, Nuciforo P, Stephan-Otto Attolini C, Albà MM, Muñoz J, Tian TV, Varela I, Vivancos A, Ramón Y Cajal S, Muñoz P, Rivas C, Abad M. pTINCR microprotein promotes epithelial differentiation and suppresses tumor growth through CDC42 SUMOylation and activation. Nat Commun. 2022 Nov 11;13(1):6840. doi: 10.1038/s41467-022-34529-6. PMID: 36369429; PMCID: PMC9652315.
- Vivancos A, Tabernero J. Circulating tumor DNA as a novel prognostic indicator. Nat Med. 2022 Nov;28(11):2255-2256. doi: 10.1038/s41591-022-02068-8. PMID: 36357679.
- Elez E, Ros J, Fernández J, Villacampa G, Moreno-Cárdenas AB, Arenillas C, Bernatowicz K, Comas R, Li S, Kodack DP, Fasani R, Garcia A, Gonzalo-Ruiz J, Piris-Gimenez A, Nuciforo P, Kerr G, Intini R, Montagna A, Germani MM, Randon G, Vivancos A, Smits R, Graus D, Perez-Lopez R, Cremolini C, Lonardi S, Pietrantonio F, Dienstmann R, Tabernero J, Toledo RA. RNF43 mutations predict response to anti-BRAF/EGFR combinatory therapies in BRAFV600E metastatic colorectal cancer. Nat Med. 2022 Oct;28(10):2162-2170. doi: 10.1038/s41591-022-01976-z. Epub 2022 Sep 12. PMID: 36097219; PMCID: PMC9556333.
- Zurita AJ, Graf RP, Villacampa G, Raskina K, Sokol E, Jin D, Antonarakis ES, Li G, Huang RSP, Casanova-Salas I, Vivancos A, Carles J, Ross JS, Schrock AB, Oxnard GR, Mateo J. Genomic Biomarkers and Genome-Wide Loss-of-Heterozygosity Scores in Metastatic Prostate Cancer Following Progression on Androgen-Targeting Therapies. JCO Precis Oncol. 2022 Jul;6:e2200195. doi: 10.1200/PO.22.00195. PMID: 35820087; PMCID: PMC9307307.
- Frigola J, Carbonell C, Irazno P, Pardo N, Callejo A, Cedres S, Martinez-Marti A, Navarro A, Soleda M, Jimenez J, Hernandez-Losa J, Vivancos A, Felip E, Amat R. High levels of chromosomal aberrations negatively associate with benefit to checkpoint inhibition in NSCLC. J Immunother Cancer. 2022 Apr;10(4):e004197. doi: 10.1136/jitc-2021-004197. Erratum in: J Immunother Cancer. 2022 Jun;10(6): PMID: 35477861; PMCID: PMC9047699.
- Tamborero D, Dienstmann R, Rachid MH, Boekel J, Lopez-Fernandez A, Jonsson M, Razzak A, Braña I, De Petris L, Yachnin J, Baird RD, Loriot Y, Massard C, Martin-Romano P, Opdam F, Schlenk RF, Vernieri C, Masucci M, Villalobos X, Chavarria E; Cancer Core Europe consortium; Balmaña J, Apolone G, Caldas C, Bergh J, Ernberg I, Fröhling S, Garralda E, Karlsson C, Tabernero J, Voest E, Rodon J, Lehtiö J. Author Correction: The Molecular Tumor Board Portal supports clinical decisions and automated reporting for precision oncology. Nat Cancer. 2022 May;3(5):649. doi: 10.1038/s43018-022-00378-x. Erratum for: Nat Cancer. 2022 Feb;3(2):251-261. PMID: 35449310; PMCID: PMC9135626.
- Tamborero D, Dienstmann R, Rachid MH, Boekel J, Lopez-Fernandez A, Jonsson M, Razzak A, Braña I, De Petris L, Yachnin J, Baird RD, Loriot Y, Massard C, Martin-Romano P, Opdam F, Schlenk RF, Vernieri C, Masucci M, Villalobos X, Chavarria E; Cancer Core Europe consortium; Balmaña J, Apolone G, Caldas C, Bergh J, Ernberg I, Fröhling S, Garralda E, Karlsson C, Tabernero J, Voest E, Rodon J, Lehtiö J. The Molecular Tumor Board Portal supports clinical decisions and automated reporting for precision oncology. Nat Cancer. 2022 Feb;3(2):251-261. doi: 10.1038/s43018-022-00332-x. Epub 2022 Feb 24. Erratum in: Nat Cancer. 2022 May;3(5):649. PMID: 35221333; PMCID: PMC8882467.
- Papakonstantinou A, Gonzalez NS, Pimentel I, Suñol A, Zamora E, Ortiz C, Espinosa-Bravo M, Peg V, Vivancos A, Saura C, Villacampa G, Oliveira M. Prognostic value of ctDNA detection in patients with early breast cancer undergoing neoadjuvant therapy: A systematic review and meta-analysis. Cancer Treat Rev. 2022 Mar;104:102362. doi: 10.1016/j.ctrv.2022.102362. Epub 2022 Feb 18. PMID: 35219090.
- Verdaguer H, Saurí T, Acosta DA, Guardiola M, Sierra A, Hernando J, Nuciforo P, Miquel JM, Molero C, Peiró S, Serra-Camprubí Q, Villacampa G, Aguilar S, Vivancos A, Tabernero J, Dienstmann R, Macarulla T. ESMO Scale for Clinical Actionability of Molecular Targets Driving Targeted Treatment in Patients with Cholangiocarcinoma. Clin Cancer Res. 2022 Apr 14;28(8):1662-1671. doi: 10.1158/1078-0432.CCR-21-2384. PMID: 35042699.
- Fernández Montes A, Élez E, Vivancos A, Martínez N, González P, Covela M, de la Cámara J, Cousillas A, Méndez JC, Graña B, Aranda E. Monitoring of RAS mutant clones in plasma of patients with RAS mutant metastatic colorectal cancer. Clin Transl Oncol. 2022 Jun;24(6):1209-1214. doi: 10.1007/s12094-021-02767-7. Epub 2022 Jan 7. PMID: 34997474; PMCID: PMC9107427.
- Prat A, Guarneri V, Pascual T, Brasó-Maristany F, Sanfeliu E, Paré L, Schettini F, Martínez D, Jares P, Griguolo G, Dieci MV, Cortés J, Llombart- Cussac A, Conte B, Marín-Aguilera M, Chic N, Puig-Butillé JA, Martínez A, Galván P, Tsai YH, González-Farré B, Mira A, Vivancos A, Villagrasa P, Parker JS, Conte P, Perou CM. Development and validation of the new HER2DX assay for predicting pathological response and survival outcome in early-stage HER2-positive breast cancer. EBioMedicine. 2022 Jan;75:103801. doi: 10.1016/j.ebiom.2021.103801. Epub 2022 Jan 3. PMID: 34990895; PMCID: PMC8741424.
- Sánchez-Guixé M, Hierro C, Jiménez J, Viaplana C, Villacampa G, Monelli E, Brasó-Maristany F, Ogbah Z, Parés M, Guzmán M, Grueso J, Rodríguez O, Oliveira M, Azaro A, Garralda E, Tabernero J, Casanovas O, Scaltriti M, Prat A, Dienstmann R, Nuciforo P, Saura C, Graupera M, Vivancos A, Rodon J, Serra V. High <i>FGFR1-4</i> mRNA Expression Levels Correlate with Response to Selective FGFR Inhibitors in Breast Cancer. Clin Cancer Res. 2022 Jan 1;28(1):137-149. doi: 10.1158/1078-0432.CCR-21-1810. Epub 2021 Sep 30. PMID: 34593528.
- Pernas S, Villagrasa P, Vivancos A, Scaltriti M, Rodón J, Burgués O, Nuciforo P, Canes J, Paré L, Dueñas M, Vidal M, Cejalvo JM, Perelló A, Llommbard-Cussac A, Dorca J, Montaño A, Pascual T, Oliveira M, Ribas G, Rapado I, Prat A, Ciruelos E. First Nationwide Molecular Screening Program in Spain for Patients With Advanced Breast Cancer: Results From the AGATA SOLTI-1301 Study. Front Oncol. 2021 Nov 4;11:744112.
- Brasó-Maristany F, Sansó M, Chic N, Martínez D, González-Farré B, Sanfeliu E, Ghiglione L, Carcelero E, Garcia-Corbacho J, Sánchez M, Soy D, Jares P, Peg V, Saura C, Muñoz M, Prat A, Vivancos A. Case Report: A Case Study Documenting the Activity of Atezolizumab in a PD-L1-Negative Triple-Negative Breast Cancer. Front Oncol. 2021 Sep 20;11:710596.
- Martinez-Marti A, Felip E, Mancuso FM, Caratú G, Matito J, Nuciforo P, Sansano I, Diaz-Mejia N, Cedrés S, Callejo A, Iranzo P, Pardo N, Miquel JM, Navarro A, Vivancos A, Sansó M. Genetic evolution to tyrosine kinase inhibitory therapy in patients with EGFR-mutated non-small-cell lung cancer. Br J Cancer. 2021 Nov;125(11):1561-1569.
- Saura C, Matito J, Oliveira M, Wildiers H, Brufksy AM, Waters SH, Hurvitz SA, Moy B, Kim SB, Gradishar WJ, Queiroz GS, Cronemberger E, Wallweber GJ, Bebchuk J, Keyvanjah K, Lalani AS, Bryce R, Vivancos A, Eli LD, Delaloge S. Biomarker Analysis of the Phase III NALA Study of Neratinib + Capecitabine versus Lapatinib + Capecitabine in Patients with Previously Treated Metastatic Breast Cancer. Clin Cancer Res. 2021 Nov 1;27(21):5818-5827.
- Ogbah Z, Mancuso FM, Vivancos A. MYC Copy Number Detection in Clinical Samples Using a Digital DNA-Hybridization and Detection Method. Methods Mol Biol. 2021;2318:321-336.
- Kagawa Y, Elez E, García-Foncillas J, Bando H, Taniguchi H, Vivancos A, Akagi K, García A, Denda T, Ros J, Nishina T, Baraibar I, Komatsu Y, Ciardiello D, Oki E, Kudo T, Kato T, Yamanaka T, Tabernero J, Yoshino T. Combined Analysis of Concordance between Liquid and Tumor Tissue Biopsies for RAS Mutations in Colorectal Cancer with a Single Metastasis Site: The METABEAM Study. Clin Cancer Res. 2021 May 1;27(9):2515-2522.
- Frigola J, Navarro A, Carbonell C, Callejo A, Iranzo P, Cedrés S, Martinez-Marti A, Pardo N, Saoudi-Gonzalez N, Martinez D, Jimenez J, Sansano I, Mancuso FM, Nuciforo P, Montuenga LM, Sánchez-Cespedes M, Prat A, Vivancos A, Felip E, Amat R. Molecular profiling of long-term responders to immune checkpoint inhibitors in advanced non-small cell lung cancer. Mol Oncol. 2021 Apr;15(4):887-900.
- Targeted multiplex proteomics for molecular prescreening and biomarker discovery in metastatic colorectal cancer. Serna G, Ruiz-Pace F, Cecchi F, Fasani R, Jimenez J, Thyparambil S, Landolfi S, Elez E, Vivancos A, Hembrough T, Tabernero J, Dienstmann R, Nuciforo P. Sci Rep. 2019 Sep 19;9(1):13568.
- Early evolutionary divergence between papillary and anaplastic thyroid cancers. Capdevila J, Mayor R, Mancuso FM, Iglesias C, Caratú G, Matos I, Zafón C, Hernando J, Petit A, Nuciforo P, Cameselle-Teijeiro JM, Álvarez CV, Recio JA, Tabernero J, Matias-Guiu X, Vivancos A, Seoane J. Ann Oncol. 2019 Nov 1;30(11):1843.
- Impact of circulating tumor DNA mutant allele fraction on prognosis in RAS-mutant metastatic colorectal cancer.Elez E, Chianese C, Sanz-García E, Martinelli E, Noguerido A, Mancuso FM, Caratù G, Matito J, Grasselli J, Cardone C, Esposito Abate R, Martini G, Santos C, Macarulla T, Argilés G, Capdevila J, Garcia A, Mulet N, Maiello E, Normanno N, Jones F, Tabernero J, Ciardello F, Salazar R, Vivancos A. Mol Oncol. 2019 Sep;13(9):1827-1835.
- Comparison of the Clinical Sensitivity of the Idylla Platform and the OncoBEAM RAS CRC Assay for KRAS Mutation Detection in Liquid Biopsy Samples. Vivancos A, Aranda E, Benavides M, Élez E, Gómez-España MA, Toledano M, Alvarez M, Parrado MRC, García-Barberán V, Diaz-Rubio E. Sci Rep. 2019 Jun 20;9(1):8976.
- Genomic heterogeneity and efficacy of PI3K pathway inhibitors in patients with gynaecological cancer. Rodriguez-Freixinos V, Ruiz-Pace F, Fariñas-Madrid L, Garrido-Castro AC, Villacampa G, Nuciforo P, Vivancos A, Dienstmann R, Oaknin A. ESMO Open. 2019 Mar 8;4(2):e000444.
- Vivancos A, Élez E, Salazar R. Circulating cell-free DNA as predictor of treatment failure after neoadjuvant chemoradiotherapy before surgery in patients with locally advanced rectal cancer: is it ready for primetime? Ann Oncol.29: 532-534.
- Capdevila J, Mayor R; Mancuso FF, Iglesias C; Caratù G, Matos I, Zafón C, Hernando J, Petit A, Nuciforo P, Cameselle-Teijeiro JM, Álvarez C, Recio JA, Tabernero J, Matias-Guiu X, Vivancos A, Seoane J. Early evolutionary divergence between papillary and anaplastic thyroid cancers. Ann Oncol.29: 1454-1460.
- Cedrés S, Felip E, Cruz C, Martinez de Castro A, Pardo N, Navarro A, Martinez-Marti A, Remon J, Zeron-Medina J, Balmaña J, Llop-Guevara A, Miquel JM, Sansano I, Nuciforo P, Mancuso F, Serra V, Vivancos A. Activity of HSP90 Inhibiton in a Metastatic Lung Cancer Patient With a Germline BRCA1 Mutation. J Natl Cancer Inst. 110: 914-917.
- Puig I, Tenbaum SP, Chicote I, Arqués O, Martínez-Quintanilla J, Cuesta-Borrás E, Ramírez L, Gonzalo P, Soto A, Aguilar S, Eguizabal C, Caratù G, Prat A, Argilés G, Landolfi S, Casanovas O, Serra V, Villanueva A, Arroyo AG, Terracciano L, Nuciforo P, Seoane J, Recio JA, Vivancos A, Dienstmann R, Tabernero J, Palmer HG. TET2 controls chemoresistant slow-cycling cancer cell survival and tumor recurrence. J Clin Invest. 128: 3887-3905.
- Cruz C, Castroviejo-Bermejo M, Gutiérrez-Enríquez S, Llop-Guevara A, Ibrahim YH, Gris-Oliver A, Bonache S, Morancho B, Bruna A, Rueda OM, Lai Z, Polanska UM, Jones GN; Kristel P, de Bustos L, Guzman M, Rodriguez O, Grueso J, Montalban G, Caratú G, Mancuso F, Fasani R, Jiménez J, Howat WJ, Dougherty B, Vivancos A, Nuciforo P, Serres-Créixams X, Rubio IT, Oaknin A, Cadogan E, Barrett JC, Caldas C, Baselga J, Saura C, Cortés J, Arribas J, Jonkers J, Díez O; O’Connor MJ, Balmaña J, Serra V. RAD51 foci as a functional biomarker of homologous recombination repair and PARP inhibitor resistance in germline BRCA-mutated breast cancer. Ann Oncol. 29: 1203-1210.
- Martínez-Ricarte F, Mayor R, Martínez-Sáez E, Rubio-Pérez C, Pineda E, Cordero E, Cicuéndez M, Poca MA, Lopez-Bigas N, Ramón Y Cajal S, Vieito M, Carles J, Tabernero J, Vivancos A, Gallego S, Graus F, Sahuquillo J, Seoane J. Molecular Diagnosis of Diffuse Gliomas through Sequencing of Cell-Free Circulating Tumor DNA from Cerebrospinal Fluid. Clin Cancer Res. 24: 2812-2819.
- Martinez-Marti A, Felip E, Matito J, Mereu E, Navarro A, Cedrés S, Pardo N, Martinez de Castro A, Remon J, Miquel J M, Guillaumet-Adkins A, Nadal E, Rodriguez-Esteban G, Arqués O, Fasani R, Nuciforo P, Heyn H, Villanueva A, Palmer H G, Vivancos A. Dual MET and ERBB inhibition overcomes intratumor plasticity in osimertinib-resistant-advanced non-small-cell lung cancer (NSCLC). Ann Oncol. 2017 Oct 1;28(10):2451-2457.
- García-Foncillas J, Alba E, Aranda E, Díaz-Rubio E, López-López R, Tabernero J, Vivancos A. Incorporating BEAMing technology as a liquid biopsy into clinical practice for the management of colorectal cancer patients: an expert taskforce review. Ann Oncol. 2017 Dec 1;28(12):2943-2949.
- Dienstmann R, Elez E, Argiles G, Matos I, Sanz-Garcia E, Ortiz C, Macarulla T, Capdevila J, Alsina M, Sauri T, Verdaguer H, Vilaro M, Ruiz-Pace F, Viaplana C, Garcia A, Landolfi S, Palmer HG, Nuciforo P, Rodon J, Vivancos A, Tabernero J. Analysis of mutant allele fractions in driver genes in colorectal cancer – biological and clinical insights. Mol Oncol. 2017 Sep;11(9):1263-1272.
- Grasselli J, Elez E, Caratù G, Matito J, Santos C, Macarulla T, Vidal J, Garcia M, Viéitez JM, Paéz D, Falcó E, Lopez Lopez C, Aranda E, Jones F, Sikri V, Nuciforo P, Fasani R, Tabernero J, Montagut C, Azuara D, Dienstmann R, Salazar R, Vivancos A. Concordance of blood- and tumor-based detection of RAS mutations to guide anti-EGFR therapy in metastatic colorectal cancer. Ann Oncol. 2017 Jun 1;28(6):1294-1301.
- Pérez-Alea M, Vivancos A, Caratú G, Matito J, Ferrer B, Hernandez-Losa J, Cortés J, Muñoz E, Garcia-Patos V, Recio JA. Genetic profile of GNAQ-mutated blue melanocytic neoplasms reveals mutations in genes linked to genomic instability and the PI3K pathway. Oncotarget. 2016 May 10;7(19):28086-95. doi: 10.18632/oncotarget.8578.
- Arqués O, Chicote I, Puig I, Tenbaum SP, Argilés G, Dienstmann R, Fernández N, Caratù G, Matito J, Silberschmidt D, Rodon J, Landolfi S, Prat A, Espín E, Charco R, Nuciforo P, Vivancos A, Shao W, Tabernero J, Palmer HG. Tankyrase Inhibition Blocks Wnt/β-Catenin Pathway and Reverts Resistance to PI3K and AKT Inhibitors in the Treatment of Colorectal Cancer. Clin Cancer Res. 2016 Feb 1;22(3):644-56. doi: 10.1158/1078-0432.CCR-14-3081.
- Ibarrola-Villava M, Fleitas T, Llorca-Cardeñosa MJ, Mongort C, Alonso E, Navarro S, Burgues O, Vivancos A, Cejalvo JM, Perez-Fidalgo JA, Roselló S, Ribas G, Cervantes A. Determination of somatic oncogenic mutations linked to target-based therapies using MassARRAY technology. Oncotarget. 2016 Apr 19;7(16):22543-55. doi: 10.18632/oncotarget.8002.
- Alves-Rodrigues I, Ferreira PG, Moldón A, Vivancos AP, Hidalgo E, Guigó R, Ayté J. Spatiotemporal Control of Forkhead Binding to DNA Regulates the Meiotic Gene Expression Program. Cell Rep. 2016 Feb 2;14(4):885-95. doi: 10.1016/j.celrep.2015.12.074.
- Vivancos A, Caratú G, Matito J, Muñoz E, Ferrer B, Hernández-Losa J, Bodet D, Pérez-Alea M, Cortés J, Garcia-Patos V, Recio JA. Genetic evolution of nevus of Ota reveals clonal heterogeneity acquiring BAP1 and TP53 mutations. Pigment Cell Melanoma Res. 2016 Mar;29(2):247-53. doi: 10.1111/pcmr.12452.
- Yus E, Güell M, Vivancos AP, Chen WH, Lluch-Senar M, Delgado J, Gavin AC, Bork P, Serrano L. Transcription start site associated RNAs in bacteria. Mol. Syst. Biol. 2012; 8: 585
- Esteve-Codina A, Kofler R, Himmelbauer H, Ferretti L, Vivancos AP, Groenen MA, Folch JM, Rodríguez MC, Pérez-Enciso M. Partial short-read sequencing of a highly inbred Iberian pig and genomics inference thereof. Heredity (Edinb) 2011 Sep; 107(3): 256-64
- Zuin A, Carmona M, Morales-Ivorra I, Gabrielli N, Vivancos AP, Ayté J, Hidalgo E. Lifespan extension by calorie restriction relies on the Sty1 MAP kinase stress pathway. EMBO J. 2010 Mar; 29(5): 981-91
- Vivancos AP, Castillo EA, Biteau B, Nicot C, Ayté J, Toledano MB, Hidalgo E. A cysteine-sulfinic acid in peroxiredoxin regulates H2O2-sensing by the antioxidant Pap1 pathway. Proc. Natl. Acad. Sci. U.S.A. 2005 Jun; 102(25): 8875-80
- Vivancos AP, Castillo EA, Jones N, Ayté J, Hidalgo E. Activation of the redox sensor Pap1 by hydrogen peroxide requires modulation of the intracellular oxidant concentration. Mol. Microbiol. 2004 Jun; 52(5): 1427-35
- Castillo EA, Vivancos AP, Jones N, Ayté J, Hidalgo E. Schizosaccharomyces pombe cells lacking the Ran-binding protein Hba1 show a multidrug resistance phenotype due to constitutive nuclear accumulation of Pap1. J. Biol. Chem. 2003 Oct; 278(42): 40565-72
- Avanzando hacia la implementación clínica: Estudio de las bases moleculares de la liberación de DNA tumoral en sangre. FIS-ISCIII. (PI20/01112). PI: Ana Vivancos. 01/01/2021 – 31/12/2023. Proyecto CPP2021 CPP2021 -009037 financiado por MCIN/AEI /10.13039/501100011033 y la Unión Europea NextGenerationEU / PRTR

- CGI-Clinics. Data-Driven Cancer Genome Interpretation for Personalized Cancer Treatment. Funding: HORIZON-HLTH-2021-CARE-05-02. HORIZON-RIA 101057509. Award period: 01/11/2022 – 31/10/2027. Principal Investigator: Ana Vivancos.
- Estudio de las bases moleculares de la liberación de DNA tumoral en sangre. FIS-ISCIII. (PI20/01112). PI: Ana Vivancos. 01/01/2021 – 31/12/2023.
- A pivotal study of derazantinib in patients with inoperable or advanced intrahepatic cholangiocarcinoma and FGFR2 gene fusions or FGFR2 gene mutations or amplifications. PI: Ana Vivancos. 22/02/2021 – 31/12/2021.
- Phase II Study of Avelumab plus chemotherapy in the peri-operative treatment for patients with resectable Gastric cancer (GC) or Gastroesophageal Junction cancer (GECJ). Merck Healthcare KGaA. PI: Ana Vivancos. 10/04/2019 – 31/12/2026.
- CeLac and European consortium for a personalized medicine approach to Gastric Cancer-LEGACy. European Commission-LEGACy. PI: Ana Vivancos. 01/01/2019-31/12/2022.
- Estudio de la microbiota, de Fusobacterium nucleatum y de la correlación con las vías de los receptores de Toll-like en tumores neuroendocrinos de intestino delgado. PI: Ana Vivancos. 21/07/2021 -3 1/12/2022.
- Olaparib and durvalumab (MEDI4736) in patients with metastatic pancreatic cancer and DNA Damage Repair genes alterations. PI: Ana Vivancos. 27/10/2021-31/12/2022.
- Evaluate the ability to detect and concordance level between FGFR2 fusions in tumor samples and cfDNA. Co-Principal Investigator: Ana Vivancos. Funding: Incyte Biosciences International. Award period: 07/10/2020 – 31/12/2022.
- Moving Liquid biopsy beyond current applications: study of prognostic and predictivevalues of circulating tumor DNA in metastatic colorectal cancer. Principal Investigator: Ana Vivancos. Funding: Asociación Española Contra el Cáncer. Reference: PROYE18031VIVA. Award period: 01/11/2018 – 31/10/2022.
- ctDNA in breast milk for early detection of pregnancy associated breast cancer. Principal investigator: Cristina Saura & Ana Vivancos. Funding: Fundación FERO – GHD. Award period: 01/07/2020-20/06/2022.
- CeLac and European consortium for a personalized medicine approach to Gastric Cancer-LEGACy. Principal Investigator: Ana Vivancos. Funding: European Commission. Award period: 01/01/2019 – 31/12/2022.
- Tumores primarios múltiples en pacientes con cáncer de pulmón: elaboración de un perfil molecular integral para elucidar orígenes genéticos comunes. Principal Investigator: Ana Vivancos. Funding: Fundación Científica de la Asociación Española Contra el Cáncer (AECC). Código proyecto: INVES19056SANS. Award period: 01/12/2019 – 30/11/2023.